New laboratory guideline optimizes diagnosis, treatment monitoring of differences of sex development
Click Here to Manage Email Alerts
The accurate quantification of peptide hormones in patients suspected of having a difference of sex development is “essential” for first-line testing and monitoring of the condition, according to a new consensus statement published in the European Journal of Endocrinology.
“Differences of sex development, or DSD, are a group of rare endocrine conditions that can be difficult to diagnose biochemically due to their complexity,” Trine Holm Johannsen, MD, PhD, a consultant and specialist in clinical biochemistry in the department of growth and reproduction at Rigshospitalet, University of Copenhagen, told Healio. “Therefore, recommendations on appropriate laboratory assessment of patients born with a DSD condition were urgently needed.”
Data from a recent international survey suggest that the diagnosis of a newborn suspected of having DSD is influenced by appropriate access to specialists, thereby resulting in a substantial variation in the initial evaluation of the child, Johannsen and colleagues wrote in the statement. The European Cooperation in Science and Technology Action “DSDnet” and the European Reference Network on Rare Endocrine Conditions developed recommendations on laboratory assessment for DSD to improve and unify optimal diagnosis and management of DSD across centers and countries. The publication is the first position paper addressing peptide hormones in relation to DSD, according to the researchers.
“This underlines the necessity of networks consisting of highly specialized laboratories with knowledge of DSD,” the researchers wrote. “The laboratory assessment of patients with known or suspected DSD requires biochemical and genetic assessment.”
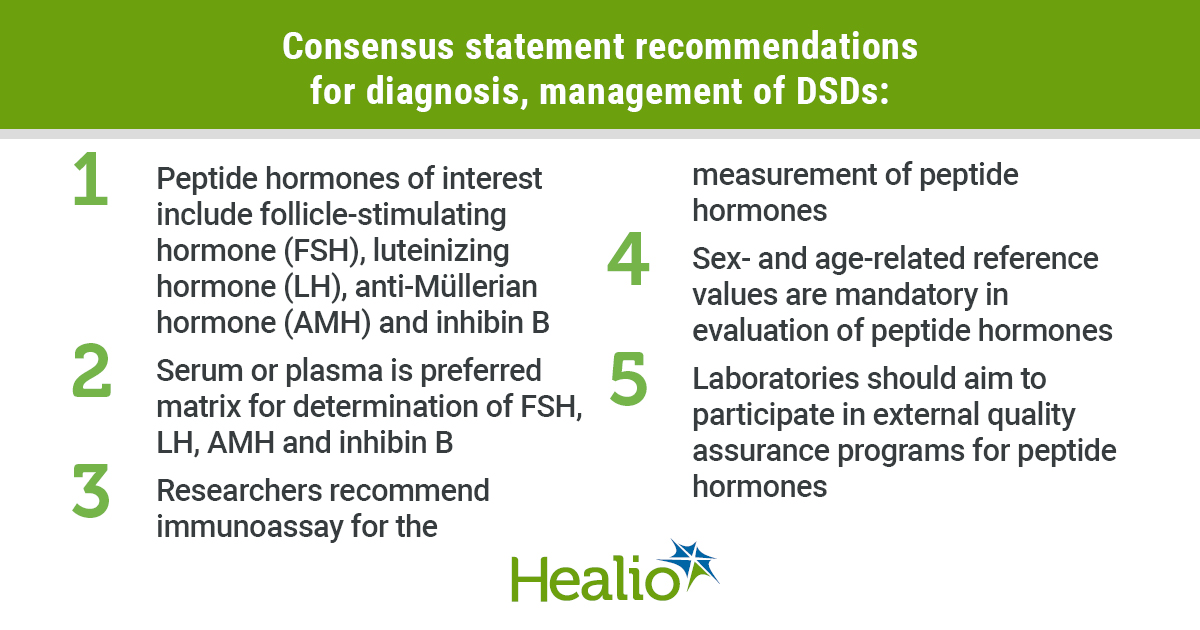
Peptide hormones include the gonadotropins follicle-stimulating hormone (FSH), luteinizing hormone (LH), anti-Müllerian hormone (AMH) and inhibin B. The request for these peptide hormones should be based on clinical examination and supplemented with relevant steroid hormones and genetic analyses, the researchers wrote.
Additionally, the statement notes that serum or plasma is the preferred matrix for the determination of LH, FSH, AMH and inhibin B; urine can be used to measure LH in experimental settings.
For diagnosis and treatment monitoring of DSD, measurement by immunoassay for FSH, LH, AMH and inhibin B are the recommended method and have been compared in accredited expert centers across Europe, Johannsen said.
“All four hormones can be measured by immunoassay while diagnostic analysis by liquid chromatography-tandem mass spectrometry awaits implementation,” Johannsen said. “External quality assurance programs are commonly accessible, apart from programs for inhibin B.”
Due to sex dimorphism and physiological fluctuations in concentrations with age and developmental stage, the researchers wrote, sex- and age-related reference values are mandatory in the evaluation of peptide hormones.
“In a diagnostic setting, both a clinical and a biochemical approach is mandatory, and knowledge of peptide hormone physiology and structure is essential,” the researchers wrote. “Five position statements have been developed to support the alignment of peptide hormone analysis in patients suspected of DSD conditions. This paper will support the establishment of common sex- and age-related references for normative data to optimize diagnostics and treatment.” – by Regina Schaffer
For more information:
Trine Holm Johannsen, MD, PhD, can be reached at the Department of Growth and Reproduction, University Hospital of Copenhagen, Rigshospitalet, Blegdamsvej 9, DK-2100, Copenhagen, Denmark; email: trine.holm.johannsen@regionh.dk.
Disclosures: The authors report no relevant financial disclosures.

